Botox Munich – Expert Wrinkle Treatment with Botulinum Toxin
Wrinkle Treatment With Botox at a Glance
- Botulinum Toxin (commonly known as Botox) is a neurotoxin that reduces muscle activity, effectively smoothing dynamic wrinkles.
- Since it is naturally broken down by the body, the effect of the treatment is temporary, typically lasting 4 to 6 months.
- The injections cause minimal discomfort, so anesthesia is usually not necessary.
- Common side effects include redness, swelling, and bruising, which usually subside within 2–3 days.
- There is no downtime after a Botox treatment. However, exercise, sauna, tanning, and intense physical activity should be avoided for about 24 hours to prevent unintended spread of the product.
- The initial session takes about 30–45 minutes, and follow-up treatments usually last 15–30 minutes.
- The cost for a wrinkle treatment in my Munich practice starts at approximately €290 (indicative, billed individually according to GOÄ, the German Medical Fee Schedule).
Table of Contents:
- What Is Botox?
- How Does a Botox Treatment Work?
- Which Wrinkles Can Be Treated With Botox?
- Treatment Procedure at My Practice
- When Does the Effect Start and How Long Does It Last?
- How Natural Does the Result Look?
- Botox or Hyaluronic Acid – What’s the Difference?
- Risks and Possible Side Effects
- Cost of Botox Treatment in Munich
- Aftercare and Follow-Up Treatments
- Botox for Special Indications
- Frequently Asked Questions (FAQ)
- Why Choose Dr. Eva Maria Strobl for Botox in Munich?
What Is Botox?
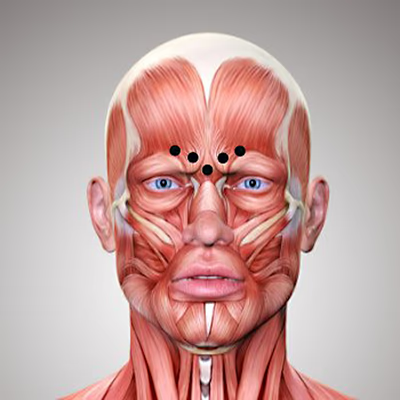
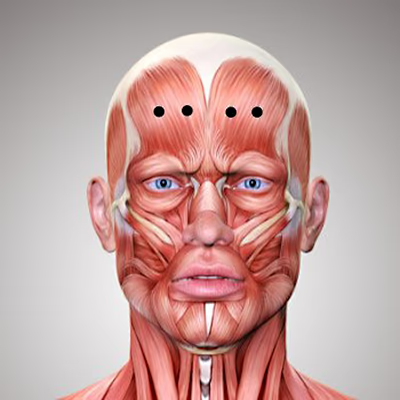
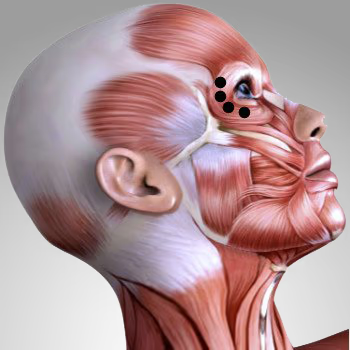
Botox (short for botulinum toxin) is one of the most widely used products in aesthetic medicine. In my Munich practice, I use Botox specifically to smooth dynamic wrinkles such as forehead lines, frown lines, and crow’s feet.
The active ingredient relaxes the treated facial muscles by temporarily blocking the signal transmission between nerve and muscle. This allows the skin to recover, appearing visibly smoother and more relaxed.
Botox is injected in minimal doses, acts only locally, and is completely broken down by the body after a few months. This makes the treatment safe, effective, and requires no downtime. Even with long-term use, Botox is considered very safe—no lasting side effects have been reported to date.
How Does a Botox Treatment Work?
Botox works by selectively blocking the transmission of nerve impulses to the muscles. Normally, when a muscle contracts, the neurotransmitter acetylcholine is released, triggering movement. Botox temporarily inhibits this release, keeping the targeted muscle relaxed. As a result, the skin above the muscle smooths out, and dynamic wrinkles such as forehead lines, frown lines, and crow’s feet become visibly reduced.
The injection is performed precisely with ultra-fine needles, usually without anesthesia, as the treatment is virtually painless. Initial results appear after about 2 to 5 days, with the full effect developing within 7 to 14 days.
The effect duration averages 3 to 6 months, after which muscle activity gradually returns. With regular treatments, the effect often lasts longer, as the muscles remain more relaxed and wrinkle formation decreases over time.
In my Munich practice, each Botox treatment is individually tailored to the patient’s facial muscles, wrinkle pattern, and desired level of naturalness in the result.
Treatment Procedure at My Practice
- Personal Consultation and Facial Analysis:
Every Botox treatment begins with a detailed consultation at my Munich practice. I analyze your facial expressions, wrinkle structure, and muscle activity to determine the precise dosage and injection points. The goal is a natural-looking result that preserves your individual facial expression. - Skin Preparation:
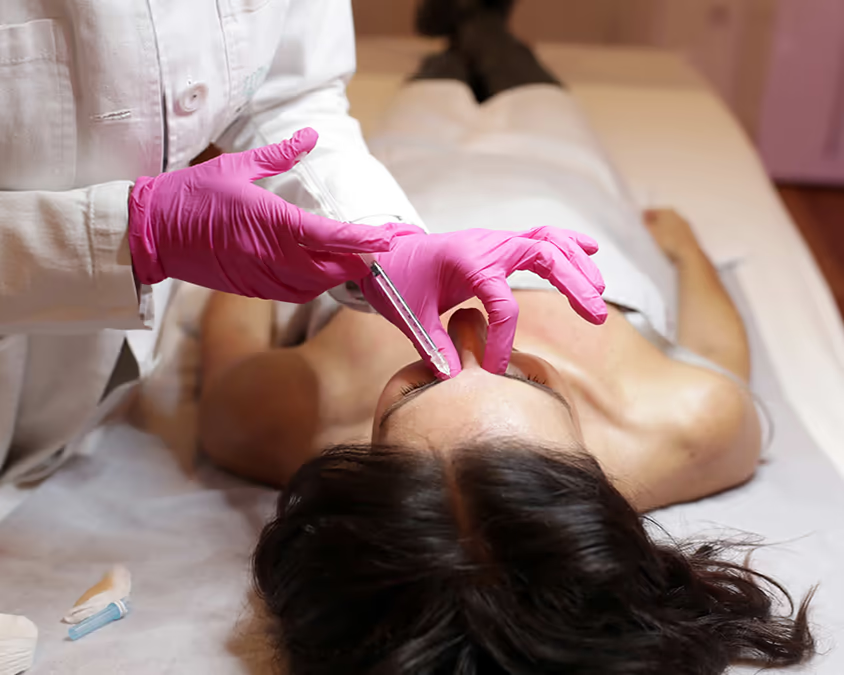
Before treatment, the skin is thoroughly cleansed and disinfected. If desired, a numbing cream can be applied for added comfort—though this is usually unnecessary, as the injections are performed with ultra-fine needles. - Precise Injection of Botulinum Toxin:
Next, Botulinum toxin type A (Botox) is injected with a micro-fine needle directly into the targeted muscles. The treatment usually takes only 10 to 20 minutes. It is an outpatient procedure, requires no anesthesia, and leaves only minimal marks, which fade quickly. - Immediate Aftercare:
Right after treatment, the skin may appear slightly red or sensitive. A brief cooling period helps soothe the area. Important: for about 24 hours, avoid exercise, sauna, and tanning, to reduce the risk of product migration. - Follow-Up and Adjustment:
The effect becomes noticeable after 2 to 5 days and reaches its maximum within one to two weeks. At my practice, I offer an optional follow-up visit after 10–14 days, where a minor touch-up can be performed if needed to refine the result.
When Does the Effect Start and How Long Does It Last?
The effect of a Botox treatment does not appear immediately but develops gradually. Initial results are usually visible after 2 to 5 days, as the treated muscles begin to relax. The full effect is typically reached after about 10 to 14 days. The skin then appears smoother, fresher, and more relaxed, without affecting natural facial expression.
The duration of effect averages 3 to 6 months. After this period, nerve-to-muscle communication gradually recovers, and muscle activity slowly returns. Regular maintenance treatments can help prolong the results, as the muscles adapt to their more relaxed state over time.
With repeated treatments, a preventive effect also develops: the formation of new expression lines is reduced, and existing wrinkles become less pronounced. At my Munich practice, I recommend individually timed Botox sessions to maintain a consistently natural and harmonious appearance.
How Natural Does the Result Look?
A natural look after Botox treatment is one of the most common concerns among my patients in Munich—they want smoother skin without a frozen or mask-like expression. This can be reliably achieved through precise dosing and targeted injection points. The goal is not to eliminate facial movement, but to relax overactive muscles responsible for unwanted wrinkles.
Your natural facial expression remains fully intact. You will look refreshed, rested, and more relaxed, yet no one will be able to tell that you’ve had Botox. With my experience in aesthetic medicine, I tailor each treatment to achieve a balanced, harmonious, and individualized result.
A core principle of my approach: start conservatively and adjust if needed, rather than overcorrect. This ensures the outcome always appears naturally beautiful and authentic.
Botox or Hyaluronic Acid – What’s the Difference?
Botox and hyaluronic acid fillers are often confused, though they have entirely different mechanisms and applications.
Botox targets the cause of dynamic wrinkles by relaxing specific muscles responsible for lines formed through repeated movement—such as on the forehead, glabellar area, or around the eyes. This smooths the skin from within, without adding volume.
Hyaluronic acid fillers, by contrast, are used for volume restoration and contouring. The gel-like substance binds water and is applied to fill static wrinkles—lines visible even without movement—or to enhance facial areas such as the lips, cheeks, or chin.
While Botox reduces muscle activity to smooth expression lines, hyaluronic acid restores firmness and fullness to the skin. In many cases, a combination treatment is ideal: Botox relaxes the muscles, and hyaluronic fillers replace lost volume—resulting in a natural, fresh, and harmonious appearance.
Because Botox carries fewer risks than hyaluronic fillers—particularly as it does not cause vascular occlusions or uneven volume distribution—I prefer it whenever it is medically and aesthetically appropriate.
At my Munich practice, I determine together with each patient whether Botox, hyaluronic acid fillers, or a combination of both best suits their skin condition and aesthetic goals.
Risks and Possible Side Effects
A professionally administered Botox treatment is considered extremely safe and is performed millions of times worldwide each year. The amounts of botulinum toxin used are minimal and act locally only. Systemic side effects are virtually nonexistent.
After injection, mild and temporary reactions may occasionally occur:
- Small redness or swelling at the injection site
- Tiny bruises
- A slight feeling of tension in the first few hours after treatment
These effects typically resolve within hours to a few days. Cooling the skin immediately afterward can further soothe the area.
In rare cases, temporary asymmetry or mild muscle weakness may occur if the product spreads into adjacent areas due to massage or pressure. For this reason, exercise, sauna, and tanning should be avoided for the first 24 hours. Such effects are harmless and fully reversible once the Botox effect subsides.
Serious complications—such as vascular occlusions or swelling reactions, which can occur with hyaluronic acid fillers—are virtually impossible with Botox. This makes it one of the safest aesthetic treatments available.
At my Munich practice, I use only original, approved products from renowned manufacturers and inject according to the highest hygienic and anatomical standards. Thanks to my extensive experience in aesthetic medicine, side effects can be minimized, ensuring a safe and natural result.
Cost of Botox Treatment in Munich
The cost of a Botox treatment depends on the area treated and the amount of product required. At my Munich practice, Botox is not billed at a flat rate per unit, but strictly according to the official German Medical Fee Schedule (GOÄ)—legally compliant and fully transparent.
For better orientation, you will find below a list of guide prices for individual treatment zones. These costs include the product, injection, consultation, and an optional follow-up appointment after treatment.
Scroll
| Botox | Price (indicative, incl. VAT) |
|---|---|
| Forehead wrinkles | 290-320€ |
| Frown line | 290-320€ |
| Forehead + Frown lines | 390-440€ |
| Crow’s feet | 290-320€ |
| Bunny lines | 290-320€ |
| Masseter relaxation | 450-480€ |
| Gummy smile | 290-320€ |
| Sagging mouth corners | 290-320€ |
| Lip lines | 290-320€ |
| Eyebrow lift | 290-320€ |
| Eyebrows Botox + PDO Lift | 680-740€ |
| Full face (2 x 50 units) | 590-640€ |
| Excessive sweating | 520-990€ |
| Chronic migraines (155-195 units) | 690-890€ |
| Micro Botox (50 units) | 390-420€ |
| Consultation | 75€ |
The prices listed in the table are indicative. The actual cost of treatment may vary—sometimes significantly—depending on your facial expression, muscle activity, and desired result. During a personal consultation, I will explain the optimal treatment plan and provide a transparent cost estimate before starting the procedure.
Aftercare and Follow-Up Treatments
After a Botox treatment, you can return to normal social activities immediately. For the first 24 hours, avoid exercise, sauna, and tanning, and do not massage or press the treated areas. Mild redness or swelling usually subsides within a few hours.
The effect typically lasts 3 to 6 months. After that, the treatment can be safely repeated to maintain long-term results. Many of my patients in Munich choose regular touch-ups two to three times per year to keep expression lines minimized and prevent new wrinkle formation.
More frequent treatments are not recommended, as they may increase the risk of antibody formation against botulinum toxin. In rare cases, this can lead to Botox resistance, where the product becomes partially or completely ineffective. Maintaining appropriate treatment intervals helps preserve long-term efficacy.
Despite precise injection techniques, minor asymmetries—such as a so-called “Spock brow”—cannot be entirely ruled out. In such cases, a follow-up appointment after about 14 days is advised to assess the result and, if needed, perform a small adjustment.
Botox for Special Indications
In addition to classic wrinkle treatment, Botox is also used for several medical and functional applications, including:

Masseter Relaxation
Botox relaxes the jaw muscles, helping to relieve jaw tension and teeth grinding while also creating a more slender facial contour.

Chronic Migraine
Botox can provide rapid relief in cases of chronic migraine. To maintain its effect, the treatment should be repeated every 12 weeks.

Excessive Sweating
Treatment of Excessive Sweating in the armpits, hands, or feet – perspiration is significantly reduced for several months.
All special applications are performed at my Munich practice in accordance with strict medical standards.
Frequently Asked Questions (FAQ)
Absolute contraindications for botulinum toxin include neuromuscular disorders such as myasthenia gravis, Lambert-Eaton syndrome, or dysphagia. Botulinum toxin must also not be administered in cases of chronic respiratory distress, during acute infections or inflammations, or during pregnancy and breastfeeding. In addition, interactions may occur with certain medications and antibiotics.
Studies have shown that an injection of 100 units of Botox/Vistabel (Allergan) contains approximately 0.73 nanograms of toxin. Note: one nanogram is one billionth of a gram. For comparison, the estimated lethal dose for a 70 kg (154 lb) adult is between 90 and 150 nanograms.
There is currently no effective antidote for botulinum toxin. Antibodies derived from horse immune serum are available and used in cases of acute botulism poisoning, but they only block the further uptake of the toxin into cells. They cannot neutralize toxin that has already entered the cells. Therefore, undesirable results from cosmetic treatments cannot be reversed while the toxin remains active.
If Botox treatments are not maintained regularly, the treated muscles and wrinkles will gradually return to their original state. The toxin’s effect typically wears off after 3 to 6 months. With long-term use, however, a certain degree of muscle atrophy can occur. The effect is similar to what happens to athletes who stop training for an extended period. After discontinuation, it may take some time for the muscles to fully recover.
There are now products from several manufacturers, all based on botulinum toxin type A. They differ mainly in protein structure and, therefore, in molecular weight, as well as in their content of human proteins (albumin). Newer products no longer contain albumin but instead use specific peptides (protein components). These factors may make one product or another more suitable in individual cases, but they do not justify labeling any single product as the “best Botox.”
Yes. Daxxify is a next-generation botulinum toxin type A product that does not contain human serum albumin. Instead, it uses a synthetic peptide stabilizer. However, Daxxify is not yet approved in the European Union. In the EU, all currently available botulinum toxin products still contain human albumin as a stabilizing agent.


About the Author:
Dr. med. univ. Eva Maria Strobl is the owner of LIPS and SKIN Aesthetic Medicine practice in Munich. She is a trained specialist in general medicine (MedUni Vienna) and has over 10 years of specialization in non-surgical aesthetic procedures. Dr. Strobl is a member of the German Society for Aesthetic Botulinum Therapy e.V. (DGBT), the German Society of Anti-Aging Medicine e.V. (GSAAM) and of Network Global Health. She publishes regularly on her blog and on DocCheck.